Aluminum anodizing is the process of creating a protective oxide layer on the surface of aluminum, which improves its durability, corrosion resistance, and aesthetic appearance. Initially, Aluminum anodizing was primarily used to improve the appearance of aluminum products. However, over time, it became apparent that anodizing also improved the durability and corrosion resistance of aluminum, leading to its widespread adoption in many industries. Advancements in anodizing technology have led to the development of new anodizing methods, making it a critical part of modern manufacturing. Read More…
Alexandria Metal Finishers provides many finishing services, one of which is anodizing. Hardcoat anodizing, aluminum anodizing, hardcoat with Teflon® plus chromic and sulfuric anodizing are some of our processes. We provide services to aerospace, commercial, electronics, medical industries and more.
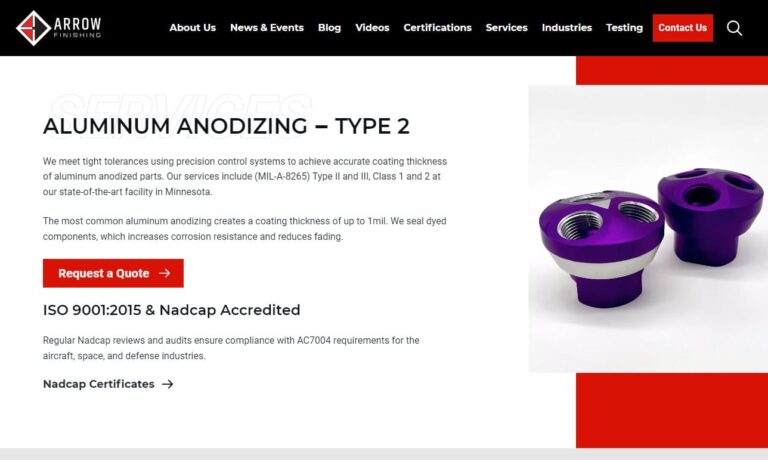
We specialize in aluminum anodizing, chromate conversion coatings, hard anodizing and sulfuric anodizing. To assure process repeatability and finish consistency, we use computer-controlled processing. All of our processes meet military specifications. Our quality system is certified to ISO 9001:2015, AS-9100 Rev D & NADCAP.
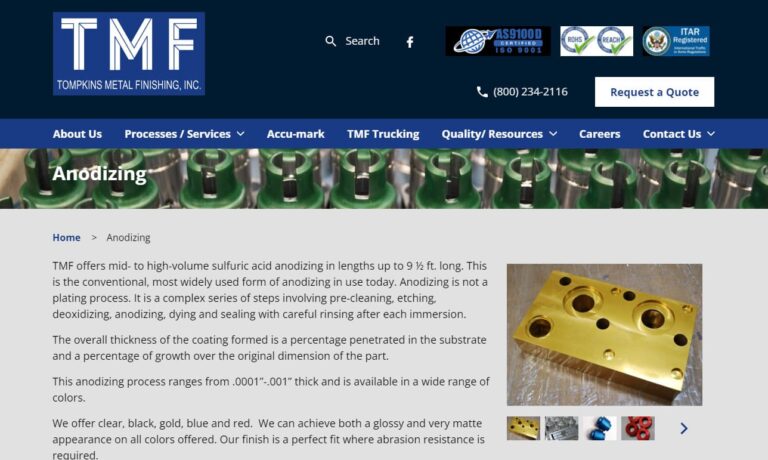
At Tompkins Metal Finishing, we offer mid to high volume aluminum anodizing. We do pre-cleaning, etching, deoxidizng, dyeing and sealing. We can achieve a wide range of surface finishes from bright to dull matte in clear, black, gold, blue and red. Other methods are also available with consideration of alloy, coating thickness, masking requirements and racking instructions.
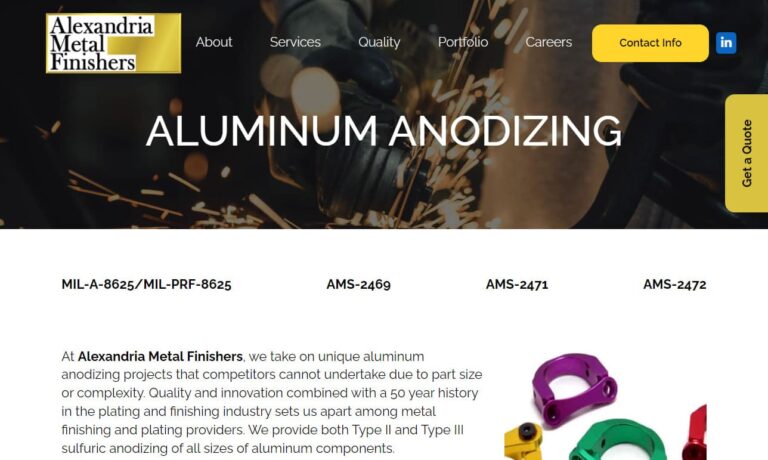
At Alexandria Metal Finishers, Inc., we are proud to offer comprehensive aluminum anodizing services tailored to the specific needs of our customers. We deliver high-quality anodized aluminum products that meet the highest standards of durability, aesthetics, and performance. Our aluminum anodizing process involves carefully preparing the surface of aluminum components and subjecting them to an...
When you choose INCERTEC, you're choosing a partner committed to excellence in every aspect of aluminum anodizing. Trust us to meet your specific requirements with a dedication to quality that goes beyond expectations. Reach out to us today for more information, and let us elevate your projects to new heights.
Dajcor Aluminum is the leading Canadian supplier of extruded, fabricated/machined and anodized components and assemblies to the automotive, renewable energy, transportation, building trades, military, recreation, and consumer-product industries.
More Aluminum Anodizing Companies
How Aluminum Anodizing Is Performed
The aluminum anodizing process is carried out by immersing the aluminum part in an electrolyte solution and passing an electric current through it. This causes the surface of the aluminum to oxidize and form a layer of aluminum oxide. There are several methods of aluminum anodizing, including sulfuric acid anodizing, chromic acid anodizing, and hard anodizing.
Sulfuric acid anodizing is the most widely used method of aluminum anodizing. The process involves immersing the aluminum part in a sulfuric acid electrolyte solution and applying a direct current. This results in the formation of a thick and hard anodic coating on the surface of the aluminum. Sulfuric acid anodizing offers excellent corrosion resistance, improved hardness, and a wide range of color options due to the ability to dye the anodic coating. However, it may not be suitable for applications where electrical insulation is required, as the coating is not highly insulating.
Chromic acid anodizing is a method used for specific applications that require a thin and highly conductive anodic coating. The process involves immersing the aluminum part in a chromic acid electrolyte solution. Chromic acid anodizing produces a thinner and softer anodic coating compared to sulfuric acid anodizing. This method offers excellent electrical conductivity and exceptional corrosion resistance. It is commonly used in the electronics industry for components that require high electrical performance. However, chromic acid is toxic and hazardous, which poses environmental and safety concerns. As a result, there are stricter regulations and limitations on the use of chromic acid.
Hard anodizing, also known as Type III anodizing, is a specialized method that produces a thicker and harder anodic coating than sulfuric acid anodizing. The process involves using a higher voltage and lower temperature compared to other methods. Hard anodizing results in a highly wear-resistant surface with increased hardness and improved corrosion resistance. It is commonly used in applications that require durability and protection, such as aerospace and military components. However, hard anodizing has limited color options, and the process may result in a darker, less decorative finish compared to sulfuric acid anodizing.
Choosing the Right Process of Aluminum Anodizing
Choosing the appropriate process for a specific application depends on several factors. Considerations include the desired properties of the anodized coating, such as corrosion resistance, hardness, and electrical conductivity. The intended application, industry requirements, and environmental regulations must also be taken into account. Sulfuric acid anodizing is a versatile and widely applicable method, suitable for most general purposes. Chromic acid anodizing is preferred for applications that require high electrical conductivity. Hard anodizing is ideal for applications that demand exceptional wear resistance and hardness.
Consulting with an experienced anodizing service provider can help determine the most suitable process based on specific requirements and constraints.
Limitations of Aluminum Anodizing
While aluminum anodizing offers numerous benefits, it is important to be aware of its limitations. One limitation is related to the size and shape of the parts that can be anodized. The anodizing process requires immersing the aluminum part in an electrolyte solution, which means that the size of the anodizing tank sets a practical limit on the dimensions of the parts that can be anodized. Complex or large-sized parts may present challenges in achieving uniform and consistent anodic coatings. Another limitation is the potential for corrosion under certain conditions. While anodizing improves the corrosion resistance of aluminum, it is not entirely impervious to corrosion. Harsh chemical environments or exposure to extreme temperatures can compromise the integrity of the anodic coating and lead to corrosion. Additionally, the cost of anodizing can be higher compared to other surface treatments. Factors such as the complexity of the part, size, and required specifications can influence the overall cost. It is important to consider these limitations and evaluate whether aluminum anodizing is the most suitable solution for specific applications, taking into account factors such as part size, environmental conditions, and budget constraints.
Efforts to Overcome These Limitations
To overcome the limitations of aluminum anodizing, efforts are being made by those in the industry to develop and implement innovative solutions. One approach is the advancement of anodizing equipment and technology. Companies are investing in research and development to enhance the anodizing process, improve process control, and achieve more uniform and consistent coatings on complex or larger parts. This includes the development of specialized anodizing tanks, improved agitation systems, and advanced monitoring and control systems. Additionally, surface pre-treatment techniques are being explored to optimize the adhesion and quality of the anodic coating, mitigating potential issues related to part size and shape. Furthermore, there is a focus on the development of new anodizing techniques and alternative electrolyte formulations to address specific limitations. For example, efforts are underway to develop environmentally friendly electrolytes that can provide improved performance while minimizing the environmental impact. These ongoing efforts reflect the commitment of the industry to overcome the limitations of aluminum anodizing and continually enhance the process to meet evolving customer needs and industry requirements.
Rules and Regulations Regarding Aluminum Anodizing
Rules and regulations play a crucial role in ensuring that aluminum anodizing processes are carried out safely and in an environmentally responsible manner. Environmental regulations aim to minimize the release of hazardous chemicals and pollutants into the environment during anodizing operations. These regulations often dictate the proper handling, storage, and disposal of chemicals used in the anodizing process. They may also require the implementation of wastewater treatment systems to remove contaminants before discharge. Additionally, workplace safety guidelines are essential to protect employees from potential hazards associated with anodizing chemicals, such as corrosive or toxic substances. These guidelines may include requirements for personal protective equipment, ventilation systems, and training programs to ensure proper handling and understanding of safety protocols. Compliance with industry standards, such as those set by organizations like the International Organization for Standardization (ISO) or local regulatory bodies, is also important to maintain the quality and consistency of anodized products. Adhering to rules and regulations is not only necessary to meet legal obligations but also to promote the health and safety of workers and minimize the impact on the environment.
Benefits of Aluminum Anodizing
Aluminum anodizing offers a range of benefits that make it a highly desirable surface treatment for aluminum products. One of the primary advantages is the enhanced surface properties it provides. Anodizing significantly improves the hardness and wear resistance of aluminum, making it more durable and capable of withstanding harsh environments. The anodic coating also acts as a protective barrier, increasing the corrosion resistance of the aluminum substrate. This prolongs the lifespan of anodized parts, reducing the need for frequent replacements. Another benefit is the versatility in color options. Anodized aluminum can be dyed to achieve a wide spectrum of colors, allowing for customization and aesthetic appeal. Moreover, anodizing does not add significant weight to the aluminum part, making it ideal for applications where weight is a critical factor. Additionally, the anodized surface is non-conductive, which can be advantageous in electrical applications where insulation is required. Another noteworthy benefit is that anodizing is a cost-effective process, particularly when compared to alternatives like plating or painting. It offers a long-lasting and low-maintenance solution, reducing overall lifecycle costs. Overall, the combination of improved surface properties, corrosion resistance, customization options, weight efficiency, electrical insulation, and cost-effectiveness makes aluminum anodizing highly advantageous in various industries.
Advantages of Aluminum Anodizing over Competing Processes
Aluminum anodizing competes with various surface treatment processes, including electroplating, painting, and physical vapor deposition (PVD) coatings. Electroplating involves depositing a layer of metal onto the aluminum surface, providing decorative and protective properties. While electroplating offers a range of finishes, it may lack the durability and hardness achieved through anodizing. On the other hand, aluminum anodizing provides superior hardness, wear resistance, and corrosion resistance compared to electroplating. Painting or organic coatings can be applied to aluminum surfaces for decorative purposes and protection against corrosion. However, compared to anodizing, these coatings may not offer the same level of durability and long-term performance. Anodizing excels in its ability to create a tightly bonded, hard, and wear-resistant oxide layer on the aluminum surface, providing enhanced durability, corrosion resistance, and a wide range of color options through dyeing. Additionally, anodizing is a more environmentally friendly process compared to electroplating or painting, as it produces minimal waste and does not involve the use of harmful chemicals. Overall, aluminum anodizing’s advantages over competing processes include superior hardness, wear resistance, corrosion resistance, aesthetic versatility, and its environmentally friendly nature.
Applications of Aluminum Anodizing
Aluminum anodizing is widely used in many industries, including:
Architecture
Anodized aluminum is commonly used for architectural elements such as windows, doors, and facades. Anodizing provides a durable finish that withstands weathering and corrosion.
Aerospace
Anodized aluminum is used extensively in the aerospace industry due to its lightweight, strength, and corrosion resistance. Applications include aircraft frames, wings, and engine components.
Automotive
Anodized aluminum is used in the automotive industry for parts such as wheels, trim, and grilles. The anodizing process improves wear resistance, corrosion resistance, and appearance.
Electronics
Due to its electrical conductivity and corrosion resistance, anodized aluminum is used in electronic devices such as smartphones and laptops.
Medical
Due to its biocompatibility and corrosion resistance, anodized aluminum is used in medical devices such as dental instruments, surgical implants, and diagnostic equipment.
Consumer Goods
Anodized aluminum is used in many consumer goods, such as cookware, sports equipment, and jewelry. The process improves wear resistance and provides an attractive finish.
Military
Anodized aluminum is used in military equipment such as guns and vehicles due to its durability, corrosion resistance, and lightweight.
Marine
Anodized aluminum is used in marine applications such as boat fittings, hulls, and masts due to its resistance to saltwater corrosion.
Packaging
Anodized aluminum is used in packaging applications such as beverage cans and food containers. The process improves corrosion resistance and provides an attractive finish.
In addition to these applications, anodized aluminum can also be used in art and architecture for decorative purposes and in the construction of electronic circuitry due to its electrical conductivity.
The Future of Aluminum Anodizing
The future of aluminum anodizing holds promising possibilities driven by advancements in technology and evolving industry demands. One area of potential development is the exploration of new anodizing methods and techniques. Research efforts are focused on improving existing processes and developing novel approaches to further enhance the properties and performance of anodized aluminum. This includes advancements in coating thickness control, surface texturing, and the development of multi-layered or composite coatings. Moreover, the development of more environmentally friendly and sustainable anodizing processes is gaining attention, with a focus on reducing energy consumption, minimizing chemical waste, and exploring alternative electrolytes. Additionally, there is growing interest in surface engineering techniques that can be combined with anodizing to achieve desired properties, such as incorporating functional nanoparticles or applying protective coatings on the anodized surface. As industries continue to seek lightweight, durable, and sustainable materials, the future of aluminum anodizing will likely involve further advancements in process efficiency, surface customization options, and expanding its applications into new sectors. Continued research and collaboration among industry experts will drive these advancements and shape the future landscape of aluminum anodizing.
Choosing the Correct Aluminum Anodizing Business
You can compare several companies using our directory of aluminum anodizing businesses. Each aluminum anodizing company has a business profile page highlighting their areas of experience and capabilities, along with a contact form to communicate with the company for more information or request a quote. Review each aluminum anodizing business using our proprietary website previewer to quickly learn what each company specializes in. Then, use our simple RFQ form to contact multiple aluminum anodizing businesses with the same form.





 Aluminum Anodizing
Aluminum Anodizing EDM
EDM Electroless Nickel Plating
Electroless Nickel Plating EMI Shielding
EMI Shielding Heat Treating
Heat Treating Metal Coating Services
Metal Coating Services Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services